In October 2022, the Tufts Center for the Study of Drug Development (CSDD) hosted an in-person executive roundtable on the correlation of CRA experience and performance.

Topics included:
- Tufts CSDD survey results on CRA experience and performance
- Factors impacting the CRA industry shortage
- Views on training, recruitment, and retention strategies
- Potential solutions to barrier on hiring CRAs with less than 2 years’ experience
- CRA recruitment and improving CRA selection to address shortages and required skills
- Challenges to the CRA role and solutions provided by organizations
- Metrics used to assess CRA performance and factors that influence CRA performance
Too learn more about the challenges and solutions, read on.
Roundtable Photos
Emmes BioPharma president Rhonda Henry
offers career advice getting into clinical research
7:10

Ken Getz
Executive Director, Tufts Center for the Study of Drug Development (CSDD)
Tufts University School of Medicine

Tufts CSDD Survey Results
Survey demographics
- 55 Respondents
- 21 Mean years of experience
- 17+ Therapeutic areas represented
- 17.5K Mean number of employees
- 91% In clinical development / operations
- 66% C -suite or head of function
Perceptions about the CRA industry shortage
- 88% There is an industry shortage
- 43% It’s getting worse
- 57% It’s staying the same
- 0% It’s getting better
CRA Education & Experience
- 44% <2 years required
- 35% 2 years required
- 21% >2 years required
- 79% Require a bachelor’s degree
- 69% Have hired a CRA with < 2 years’ experience
Training Requirements
- 62% College / university
- 59% Company
- 33% 3-4 months of training
- 18% 1-2 months of training
- 15% 5-6 months of training
- 15% < 1 month of training
- 84% Peer support / mentoring most effective training
- 77% In person most effective training
- 39% Experiential training most effective training
General areas of competency
- Scientific concepts and research design
- Ethical and participant safety considerations
- Ethical and participant safety considerations
- Investigational product development and regulation
- Clinical study operations (good clinical practice)
- Study and site management
- Data management and technology
- Leadership and professionalism
- Communication and teamwork
Technical skills needed
- Digital and remote solutions
- Data literacy
- Project management
- Risk identification and mitigation development
- Writing
Soft skills needed
- Ability to escalate
- Communication skills
- Influencing and negotiating
- Multitasking
- Organization
- Problem solving and strategic thinking
- Professionalism
- Resilience
- Building site relationships
- Managing stress
Recruiting approaches used
- 86% Recruiters
- 69% Website / social media
- 59% Staffing companies
Recruiting approaches that are most successful
- 52% Recruiters
- 38% Internal staff referrals, recommendations, self-application
Average annual turnover rate
- 25% 15-20% turnover
- 22% ≤ 10% turnover
- 16% 20-25% turnover
- 13% 10-15% turnover
Reasons for leaving
- 74% Better salaries
- 45% Better growth opportunities
- 29% Burnout
Retention strategies
- 65% Flexible work schedules
- 55% Higher salaries
- 42% Sign-on bonus
- 42% Enhanced benefits
Factors influencing CRA performance
- 40% Type of training received
- 35% Years of experience
- 10% Mentoring received by senior CRAs
- 10% Developing expertise outside of training
Challenges and solutions (partial)
| Challenges | Solutions |
|---|---|
| Turnover | Enhanced training; build CRA community engagement |
| Complicated systems / ineffective training | Simplified SOPs; focus on what matters |
| Prep, close-out & other documentation | Explore ways to automate |
| Customer service | Live interactive virtual workshop on customer experience |
| Complex protocols | More therapy / disease training |
| Changing environment | Equip CRAs with right training, processes, technology |
Source: Tufts Center for the Study of Drug Development (CSDD) CRA Executive Roundtable, October 2022
Clinical trial innovation expert
Craig Lipset shares his perspective
6:56

Dr. Gregg Collins
Chief Learning Scientist
NIIT

Experiential Training Through Spiral Learning


- People are natural learning machines, but we tend to teach in ways that are highly unnatural
- We often take something fun and interesting and made it painful and boring
- We can do better by making formal learning that works like natural learning
- We can do that by paying attention to what learning science has to tell us:
- People learning by doing, not by being told
- Learning happens when you make mistakes and get feedback
- Emotional impact makes learning stick
- Spiral learning: working through similar tasks at increasing complexity levels maximizes learning efficiency
- Critical mistake analysis: effective learning confronts learners with real-world challenges
- Surprise —unexpected events are remembered better
- Stakes —important goals command more engagement
- Consequences —high impact outcomes are remembered better
- Difficulty —hard challenges are remembered more; overcoming them drives confidence
- Empathy —human connections drive engagement
- Reality —recognition drives engagement
- Pilot training, which is highly effective, is most analogous to what effective CRA training could be:
- Spiral learning—concepts are taught gradually / repeatedly with each encounter building on earlier ones
- Critical mistake analysis—identify maximum impact mistakes novices make on the job and design challenges around them
- Simulation—virtual practice around scenarios that leads to better real-life outcomes (e.g., managing adverse events)
- Summary:
- Stop lecturing
- Create scenarios that evoke people’s learning mechanisms
- Make it hard
- Make it surprising
- Create emotional impact through storytelling
- Compress training time by targeting critical mistakes
- Avoid siloing
- Use spiral learning to ensure spaced practice
- Keep ramping up the challenge level
- The way pilots are trained is a good model to consider for more effective CRA training
Clinical trial analytics expert
Moe Alsumidaie discusses his research on CRA performance
14:22

Moe Alsumidaie
Head of Research, Clinibiz and Chief Data Scientist
Annex Clinical

Cra Experience Vs. Performance
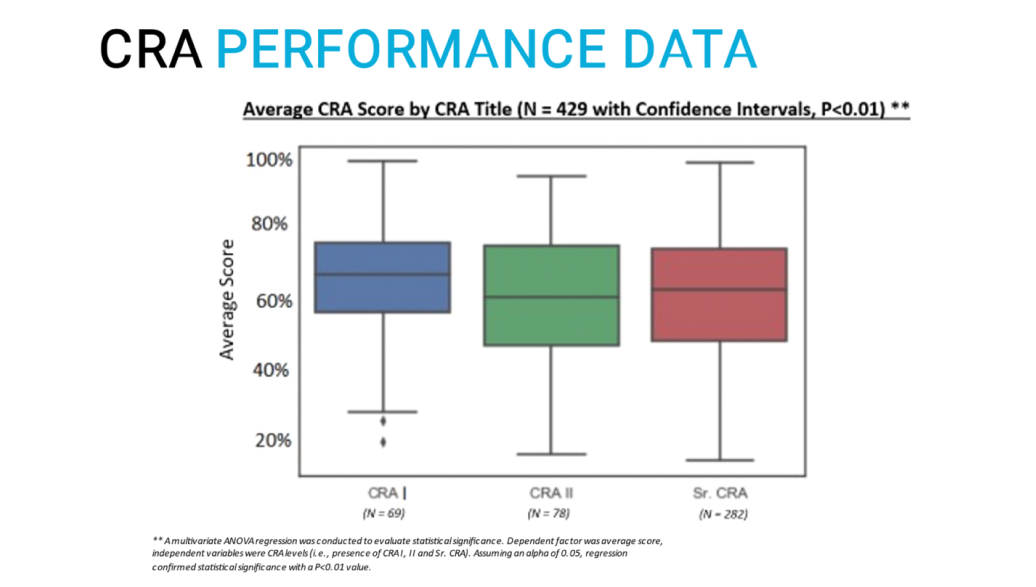


- We studied 579 CRAs with varying degrees of experience within global CROs in geographies across the world
- They received a mock study and monitoring visit scenario via simulation to capture monitoring issues
- Across all experience categories, the average score difference was not statistically significant:
- CRA experience does not appear to be associated with quality in an objective test
- CRA experience does not appear to be associated with consistent results
- Perhaps “CRA experience” is not a good performance predictor
- CCRAs may be getting promoted due to experience and not performance
- Regulations on training and experience (ICH-E6-R2):
- They don’t focus on years of experience a monitor must have
- They say that mnitrs “should be qualified by education, training, and experience”
- They “should be appropriately trained, and should have the…clinical knowledge needed to monitor”
- They “should be thoroughly familiar with the investigational product(s), the protocol, written informed consent form…”
- It’s time to rethink the two-year research experience requirement
- CRAs move into higher positions because of their experience
- The two-year barrier blocks qualified professionals with exemplary credentials to become entry-level CRAs
- CRA siphoning and entry barriers contribute to the shortage
- A better model for hiring CRAs:
- Those with the necessary clinical education and experience (including outside clinical research
- Experiential on-site support (in-person mentoring to support experience requirements, and technology
- Experiential training curriculum in ICH-GCP
- Regular performance testing for monitoring quality and consistency
Society for Clinical Research Sites leader
David Vulcano comments on the clinical trial talent shortage
10:12










