In this article and video, Applied Clinical Trials blogger and Head of Research for CliniBiz Moe Alsumidaie, MBA, MSF, takes a deep dive into the reasons behind the CRA shortage and how industry can proactively address this problems.
In 2016, there was much buzz around the talent gap impacting entry-level clinical research positions within the biopharmaceutical industry. ACRP’s position paper on the clinical research associate (CRA) shortage and ACRP helping to address the gap with its Joint Task Force for Clinical Trial Competencies raised awareness around the problem. ACRP’s position paper also highlighted the industry’s expectation of a two-year minimum experience requirement for entry-level CRA positions. Still, to this day, word on the street is that the shortage remains as CRA demand increases, and entry-level talent remains low. This article will evaluate why the shortage still exists and explore options that could address the shortage.
Why is There an Entry-Level CRA Shortage?
After researching the literature and conducting data analyses, I’ve discovered four main themes contributing towards the entry-level CRA shortage:
- Many Sponsors Require CRAs to Have a Minimum of Two-Years’ Experience – It is no secret that the industry has gone by a two-year minimum experience standard to place CRAs on their trials. One would think CRAs would be young and enthusiastic professionals and go straight into research jobs, but the average age of a CRA is 44 years old, with 10% being 20-30 years old, 27% being 30-40 years old, and 63% being over 40. This may be the case because clinical research is not a well-evangelized career path out of college. Many professionals zig-zag through different life sciences careers before becoming aware of a career in clinical research, landing a clinical research job, and gaining the experience necessary to become a CRA. This is also especially tough on CROs because sponsors do not want inexperienced CRAs to work on their trials, resulting in CROs allocating too many trials and more complex studies to their already strained workforces and causing CRA burnout. Another possibility for this age trend is the data source, as the reference data may include roles for both junior and senior CRAs, with the majority of CRAs falling in the senior level (from having years of experience).
- High Turnover: CRAs Move on to Other Roles or More Senior Roles – The average total turnover rate for CRAs is roughly 20%, and 48% of CRAs stay in their positions for 1-2 years before changing jobs. Many CRAs start at CROs, and those positions are especially prone to high turnover – primarily due to salary expectations, as compensation levels remained the same for the past decade and CRAs switch companies to attain higher salaries. Additionally, some CRAs do not have a clear career path within their organizations, leading to them leaving their positions for more senior roles elsewhere, and do not feel appreciated in their existing roles.
- Growing CRA Jobs and Low Unemployment Rates– It is no surprise that since CRAs are in such high demand, they are almost always employed; in 2019, the CRA unemployment rate was 2.5%, and CRA jobs continually increase. According to ACRP, the compounded annual growth rate (CAGR) for CRA jobs from 2016-2019 is 5.3%.
- Clinical Trial Complexity and Growth: Clinical trials and eClinical technology systems are putting more strain on CRAs, as today’s studies are becoming much more complex, and the sheer number of clinical trials is increasing (a 10% CAGR), also contributing to increasing CRA resource strain and burnout in the existing workforce.
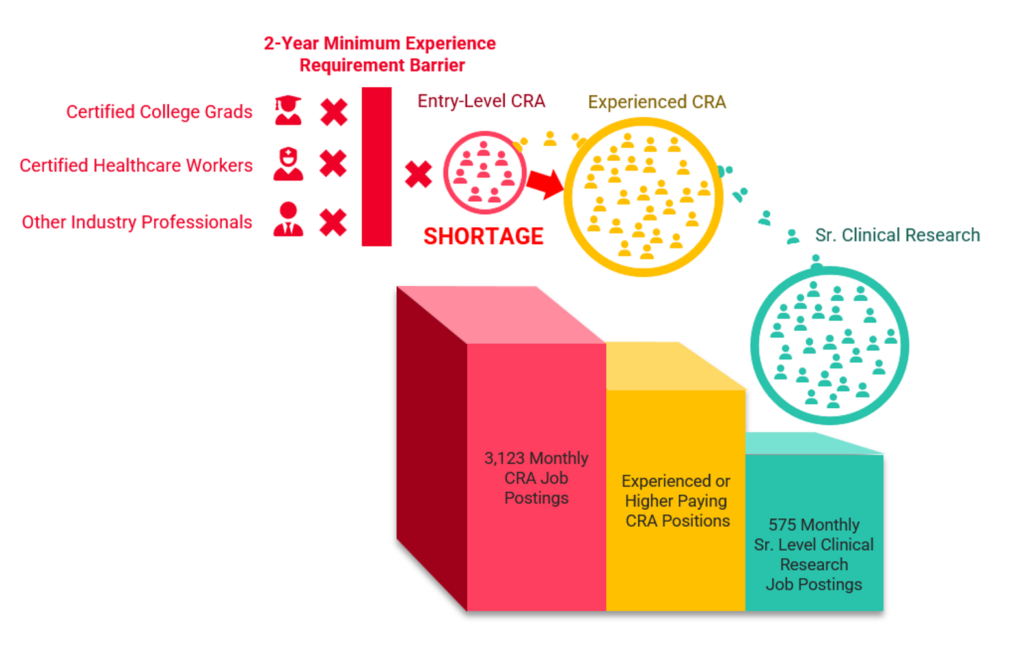
This evidence draws my perspective to the factors causing the entry-level CRA shortage: CRA entry barriers and CRA siphoning. Figure 1 demonstrates the CRA shortage problem; increasing job postings coupled with the two-year minimum experience requirement barrier prevents those jobs from being filled, and other CRA job postings and senior clinical research job postings requiring experienced CRAs are siphoning CRAs from entry-level positions. The combination of CRA entry barriers and the siphoning of CRAs to other roles coupled with entry-level CRA job growth are creating the entry-level CRA shortage.
Figure 1: The Entry-Level CRA Shortage (CRA Job Posting Numbers: ACRP)
Why the Two-Year Minimum Experience Barrier?
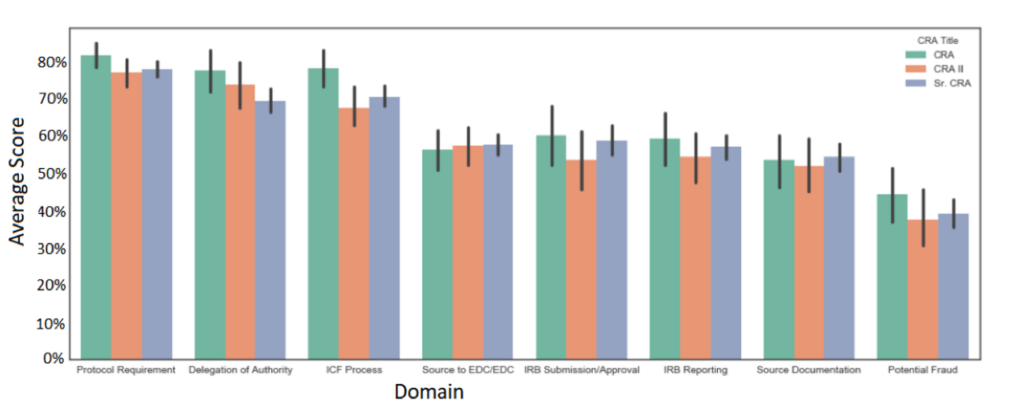
The industry follows ICH-E6-R2 guidelines when it comes to clinical trial professional expectations. It is important to note that the ICH-E6-R2 does not define how many years of experience are required or what CRA experience is; the industry has evolved to a two-year minimum experience preference. ICH-E6-R2 repeatedly mentions that personnel “should be qualified by education, training and experience.”All CRAs must train and have an education, which is accessible; however, is ‘experience’ also a proper guide to performance? I believe sponsors may equate CRA experience with performance and quality; however, data shows that CRA experience is not related to CRA performance and quality. I recall conducting a data analysis showing a minimal difference in quantified CRA performance, regardless of CRA seniority and years of experience. The analysis also revealed that CRAs’ most competent skills involved critical clinical trial competencies, such as protocol requirements, informed consent, and delegation of authority. Other skill areas where CRAs lacked involve competencies that can now be tracked and caught by quality management and technology systems, such as IRB reporting (via CTMSs) and potential fraud (via statistical RBM). One of the most critical skills CRAs lack involves source documentation, which, in my experience, requires more expertise and thought process than experience; despite my opinion, the data shows all CRAs demonstrated lower competencies in that area, regardless of seniority and experience levels.
Figure 2: Average Domain Scores by CRA Title (N= 429 with Confidence Intervals)
CRAs are trained to monitor clinical trials using consistent methodologies during CRO bootcamps. While unique perspectives gained from experience can add value (especially in a managerial role), the analysis mentioned above clearly shows that in a CRA role, experience makes a minimal difference in performance when assessed in a standardized environment, which seems to correlate with industry thought leader opinions that two years of CRA experience is not equivalent to CRA performance. Additionally, while there is some consistency with training programs, the CRA shortage issue continues to balloon, indicating that existing programs and methodologies are not addressing the root cause of the shortage, which is to fulfill the ‘experience’ requirement.
Can CRA Training Programs Include Required “Experience?”
Most clinical research professionals know the CRA boot camps at large CROs, which provide the training competency required by ICH-E6-R2 and offer some form of standardization to become a CRA. However, few sponsors will allow the placement of an inexperienced CRA on their trial unless they have two years of experience. While ICH-E6-R2 mentions the experience requirement, can’t newcomers to the industry who have educational and training requirements also undergo an experiential process? Many professions incorporate experience in their training programs; physicians go through fellowships and residencies, pharmacists go through rounds, medical assistants go through internships. Why can’t entry-level CRAs also have an experiential aspect to their training acceptable to sponsors as an industry standard?
Some training enterprises are turning this gap into an opportunity. For example, Virb, a startup company, is attempting to address this gap by raising awareness about the clinical trial profession to students nearing college graduation at universities,and administering gold-standard CRA training to recent graduates. Virb has gone further by employing a CRA performance oversight program after the CRA has been hired. “We are embedding an experiential core into our training using advanced ed tech to simulate real-world experience, real-time coaching, mentoring, and experts-on-call to overcome the lack of years in the role,” says Garrett Walker, CEO of Virb.Moreover, non-profits, such as ACRP, launched a variety of programs and taskforces to define CRA training requirements for the industry to follow, though the performance and impact of these programs on the CRA shortage is unknown.
What Will It Take to Change the Two-Year Minimum Experience Requirement?
While many factors identified in this article are contributing to the CRA shortage, addressing the two-year minimum experience requirement will bring significant relief to the problem. Perhaps it would take newly issued guidance from ICH-E6-R2 to clarify their definition of ‘experience,’ or maybe they kept the definition vague for a reason and will maintain its position. Either way, the industry-wide CRA shortage is self-inflicted because of an arbitrary number of ‘years of experience’ that has descended through the years by the industry as a ‘standard,’ because ‘we’ve always done it that way.’
COVID caused a significant shift in the industry’s clinical operational methods and broke through ossified conceptions of how trials should be run by decentralizing them; however, COVID was a crisis that demanded immediate action. While the CRA shortage may feel benign at the moment, it will snowball into a crisis if the industry does nothing to change the CRA entry barriers. Let’s face it; the issue was discussed in detail in 2016, yet, the problem persists. Perhaps in the future, the CRA shortage will become so painful to the industry that there will be no choice but to accept novel approaches to remove the entry barriers to meet ‘training, education and experience’ requirements and fill the shortage. However, it would take a culture shift and some giants, such as big CROs, to remove this roadblock since they have the capital and credibility to change industry perceptions. For certain, whichever CRO figures out this problem first will realize significant benefits, such as added revenue (due to increased capacity), advancing more studies, and moving to become the leading brand in the industry.
Moe Alsumidaie has received funding from Virb to write this article.